Compound( ZnO)

Chemical formula : ZNO
Chemical family : power
PHYSICAL / CHEMICAL DATA:
Physical state: granule , compound (masterbach)
Properties & Use: Antibacterial & UV protection
Appearance: White (cream)
Odor: None
Concentration: Pure
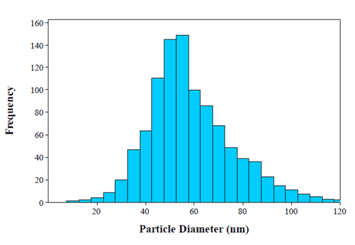
Average particle size (APS) : 40 – 80 nm
Product features: LCD , Optical films , Cosmetic material , paint .textile , coating & resin , plastic , films , PP , PE , PET , PA
FIRE OR EXPLOSION HAZARD:
Condition of flammability: product is not flammable
Flash point: Not applicable
Hazardous combustion products: No, this materials shipping specification are not classified as hazardous
REACTIVITY DATA:
Stability: Decomposition will not occur if used and stored according to specification
Conditions to avoid: e
Materials to be avoided: Alkaline metals , Alkaline earth metals
Hazardous decomposition products: None
EMERGENCY FIRST AID PROCEDURES:
Skin: Flush the contact area with lukewarm running water for at least 10 minutes. Remove contaminate clothing, taking care not to spread the chemical .if contamination is extensive, remove clothing under running water. Discard or decontaminate under running water.
Eye: Flush the contaminate eye (s) for at least 10 minutes with lukewarm running water. Holding the eyelids open. Take care not to rinse contaminated water into the non-affected eye. Always seek medical attention for accidents involving the eyes.
Ingestion: Never give anything by mouth if victim is rapidly losing consciousness, or is unconscious or convulsing. Rinse mouth thoroughly with water. Do not induce vomiting. Have victim drink 200 – 400 ml or water to dilute.
OTHER INFORMATION:
Employers should use this information only as a supplement to other information gathered by them, and should make independent judgment of suitability of this information to ensure proper use and protect the health and safety of employees. This information is furnished warranty, and any use of the product not in conformance with this Material Safety Data Sheet, or in combination with any other product or process, is the responsibility of the user.
MIXING
Instructions :
Dependence of your application and industry. But usually use 3% wt
APPLICATI
Instructions:
Adsorption
Attenuation of ultraviolet light
Cosmetics and cosmeceuticals
Demilitarization of chemical and biological warfare agents
Dental cements
Electrodes for solar cells
Environmental remediation
Flame retardant
Gas sensors
High-temperature lubricant in gas turbine engines
Photocatalytic decontamination
Piezoelectrics
Pigments for paints
UV protection
Varistors
STORAGE
Store in cool, dry place
PROPERTIES
Zinc oxide is an inorganic compound with the formula ZnO. It usually appears as a white power, nearly insoluble in water. The power is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber (e.g., car tires), lubricants, paints, ointments, adhesives, sealants, pigments, foods (source of Zn nutrient), batteries, ferrites, fire retardants, first aid tapes, etc. ZnO is present in the Earths crust as the mineral zincite; however, most ZnO used commercially is produced synthetically.
In materials science, ZnO is often called a II-IV semiconductor because zinc and oxygen belong to the 2nd and 6th groups of the periodic table, respectively. This semiconductor has several favorable properties: good transparency, high electron mobility, wide bandgap, strong room-temperature luminescence, etc. those properties are already used in energy-saving or heat-protecting windows, and electronic applications of ZnO as thin-film transistors and light-emitting diodes are forthcoming as of 2009.
Mechanical properties
ZnO is a relatively soft material with approximate hardness of 4.5 on the Mohs scale. Its elastic constants are smaller than those of relevant III-V semiconductors, such as GaN. The high heat capacity and heat conductivity, low thermal expansion and high melting temperature of ZnO are beneficial for ceramics.
Among the tetrahedrally bonded semiconductors, it has been stated that ZnO has the highest piezoelectric tensor or at least one comparable to that of GaN and AlN. This property makes it a technologically important material for many piezoelectrical applications, which require a large electromechanical coupling.
Electronic properties
ZnO has a relatively large direct band gap of ͂3.3 eV at room temperature; therefore, pure ZnO is colorless and transparent. Advantages associated with a large band gap include higher breakdown voltages, ability to sustain large electric fields, lower electronic noise, and high-temperature and high-power operation. The bandgap of ZnO can further be tuned from ͂3-4 eV by its alloying with magnesium oxide or cadmium oxide.
Most ZnO has n-type character, even in the absence of intentional doping. Nonstoichiometry is typically the origin of n-type character, but the subject remains controversial. An alternative explanation has been proposed, based on theoretical calculation, that unintentional substitutional hydrogen impurities are responsible. Controllable n-type doping is easily achieved by substituting Zn with group-III elements such as Al, Ga, in or by substituting oxygen with group-VII elements chlorine or iodine.
Reliable p-type doping of ZnO remains difficult. This problem originates from low solubility of p-type dopants and their compensation by abundant n-type impurities. This problem is observed with GaN and ZnSe. Measurement of p-type in "intrinsically" n-type material is complicated by the inhomogeneity of samples.
Current limitauons to p-doping do not limit electronic and optoelectronic applications of ZnO, which usually require junctions of n-type and p-type material. Know p-type dopants include group-I elements Li, Na, K; group-V element N, P and As; as well as copper and silver. However, many of these form deep acceptors and do not produce significant p-type conduction at room temperature.
Electron mobility of ZnO strongly varies with temperature and has a maximum of ͂2000 cm2/ (V.s) at 80K. data on hole mobility are scarce with values in the range 5-30 cm2/ (V.s).
Chemical properties
ZnO occurs as white power known as zinc white or as the mineral zincite. The mineral usually contains a certain amount of manganese and other elements and is of yellow to red color. Crystalline zinc oxide is thermochromic. Changing from white to yellow when heated and in air reverting to white on cooling. This color change is caused by a very small loss of oxygen at high temperatures to form the non-stoichiometric Zn1+xO,
where at 800˚C, x = 0.00007.
Zinc oxide is an amphoteric oxide. It is nearly insoluble in water and alcohol, but it is soluble in (degraded by) most acids, such as hydrochloric acid.